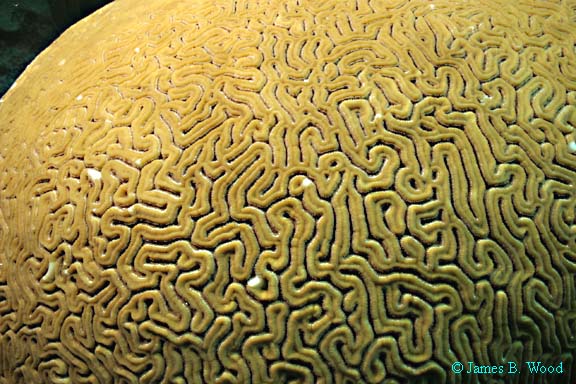
Grooved
Brain
Coral
(Diploria labyrinthiformis)
(Diploria labyrinthiformis)
By Kate
Rossi-Snook
and
Dr. James B. Wood and Kim Zeeh - Editors
and
Dr. James B. Wood and Kim Zeeh - Editors
|
Grooved
Brain
Coral
(Diploria labyrinthiformis) By Kate
Rossi-Snook
and Dr. James B. Wood and Kim Zeeh - Editors |